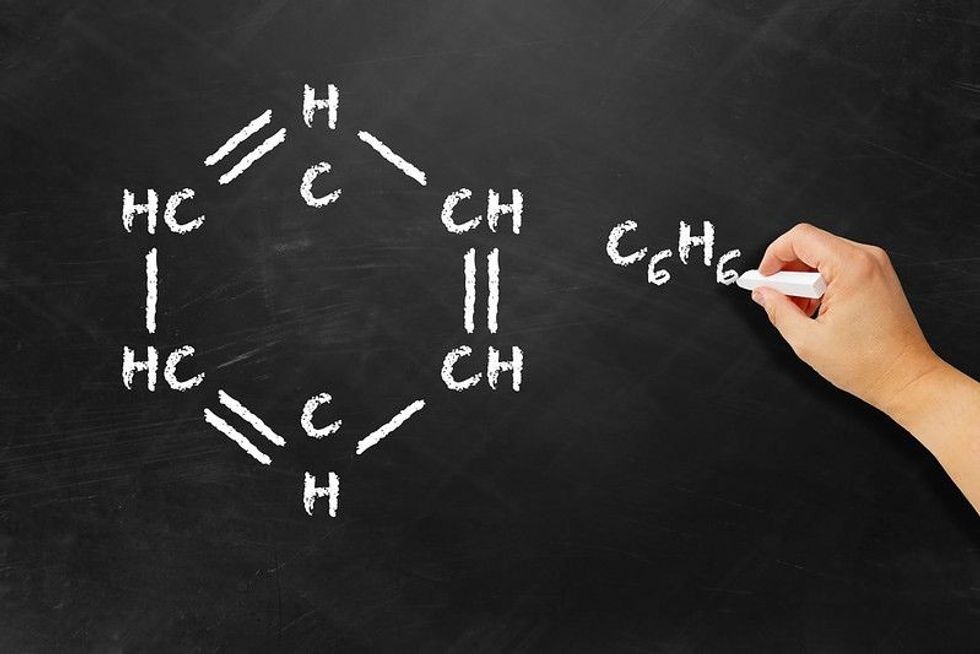
146 Carbon Chain Names To Help Your Chemistry Studies
The International Union of Pure and Applied Chemistry (IUPAC) is an international body that represents chemistry and related sciences and technologies.
It was established in 1919 and is recognized as an international authority on chemical nomenclature, UPAC rules, molecular formula, symbols, terminology, general formula, number, name, and other related aspects. The IUPAC carbon chain names help you to identify and recognize the properties of different carbon compounds.
The hydrocarbons containing double or triple bonds are known as alkenes or alkynes. IUPAC rules use the prefix 'di', 'tri', 'tetra', etc, as per the number of carbon atoms present in the carbon parent chain name. Carbon chains can also form multiple bonds.
Some of the popular carbon chain names are eth-ane, hept-ane, H 2 O, prop-ane, meth-ane, dec-ane, hex-ane, oct-ane, -ane to -yl, butane (C 4 H), and others. Alkanes are one of the functional groups also known as an alkyl group.
They get their names based on the total number of carbons that are present in the longest chain they have. All alkanes with five or more carbons make use of prefixes that point towards the number of carbons they have. Cycloalkanes again only contain carbon-hydrogen bonds and carbon-carbon single bonds.
Alkyl group formation is completed by removing one hydrogen from the alkane chain. It is mentioned by the formula, C n H 2n +1
Did you know you must give the number and name of the side chains before naming the root chain alkanes! The smaller of the two numbers designating the carbon atoms of the triple bonds are used as the triple bond locator.
All of the basic organic compounds are made up of carbon and hydrogen atoms. Hydrocarbons are made up of carbon and hydrogen.
IUPAC Carbon Chain Names
IUPAC carbon chain names helps you to learn and understand more about carbon chain common names. The first 20 alkanes starting from methane to icosane are included in the list.
As per IUPAC, the names for simple alkyl groups are provided in the table under common alkyl groups. The prefixes are added as per alphabetical order. The alphabetical order would apply to each individual group.
The most important three-carbon chain names are listed below:
Methane is the simplest hydrocarbon containing one carbon atom and four hydrogen atoms. It is a powerful greenhouse gas. Methane is also a potent heat absorber.
Ethane is a gaseous and colorless hydrocarbon containing two carbon atoms and six hydrogen atoms. Ethane has the simplest carbon-carbon bond structurally.
Propane is another gaseous hydrocarbon containing three carbon atoms and eight hydrogen atoms. Propane is stored under pressure inside a tank. It is a colorless and odorless liquid.
Long Carbon Chain Names
When we look into the carbon chain names, it is very important to understand long carbon chain names. Carbon chain root names and carbon side chain names are very important in our daily life. Go through the names of some long carbon chain names and explore them.
Butane, which has four carbon atoms, is a colorless gas and has a faint petroleum-like odor.
Decane consists of 10 carbon atoms. Decane is an important component in gasoline and kerosene.
Docosane is one of the longest chains, which has 22 carbon atoms.
Dodecane is one of the carbon-hydrogen chain names that consists of 12 carbon atoms.
Doheptacontane contains 72 carbon atoms.
Dohexacontane contains 62 carbon atoms.
Dononacontane has 92 carbon atoms.
Dooctacontane contains 82 carbon atoms.
Dopentacontane contains 52 carbon atoms.
Dotetracontane has 42 carbon atoms.
Dotriacontane has 32 carbon atoms.
Heneicosane is a straight-chain saturated hydrocarbon with 21 carbon atoms.
Henheptacontane has 71 carbon atoms.
Henhexacontane has 61 carbon atoms.
Hennonacontane consists of 91 carbon atoms.
Henoctacontane has 81 carbon atoms.
Henpentacontane contains 51 carbon atoms.
Hentetracontane contains 41 carbon atoms.
Hentriacontane consists of 31 carbon atoms.
Heptacontane is a 70 carbon-containing hydrocarbon. It is a saturated hydrocarbon that has no carbon-carbon multiple bonds.
Heptacosane consists of 27 carbon atoms.
Heptadecane consists of 17 carbon atoms.
Heptaheptacontane has 77 carbon atoms.
Heptahexacontane has 67 carbon atoms.
Heptane is another hydrocarbon with the carbon atom number being seven.
Heptanonacontane consists of 97 carbon atoms.
Heptaoctacontane consists of 87 carbon atoms.
Heptapentacontane has 57 carbon atoms.
Heptatetracontane has 47 carbon atoms.
Heptatriacontane contains 37 carbon atoms.
Hexacontane consists of 60 carbon atoms. Hexacontane is a solid paraffin hydrocarbon.
Hexacosane has 26 carbon atoms.
Hexadecane contains 16 carbon atoms.
Hexaheptacontane is one of the longest carbon chain, which contains 76 carbon atoms.
Hexahexacontane contains 66 carbon atoms.
Hexane is a hydrocarbon with six carbon atoms.
Hexanonacontane contains 96 carbon atoms.
Hexaoctacontane contains 86 carbon atoms.
Hexapentacontane contains 56 carbon atoms.
Hexatetracontane contains 46 carbon atoms.
Hexatriacontane contains 36 carbon atoms.
Icosane contains 20 carbon atoms. It is the shortest compound found in paraffin waxes and is used to form candles.
Nonacontane contains 90 carbon atoms.
Nonacosane contains 29 carbon atoms.
Nonadecane contains 19 carbon atoms.
Nonaheptacontane contains 79 carbon atoms.
Nonahexacontane contains 69 carbon atoms.
Nonane has nine carbon atoms. Nonane is a colorless and flammable liquid.
Nonanonacontane contains 99 carbon atoms.
Nonaoctacontane contains 89 carbon atoms.
Nonapentacontane has 59 carbon atoms.
Nonatetracontane has 49 carbon atoms.
Nonatriacontane contains 39 carbon atoms.
Octacontane contains 80 carbon atoms.
Octacosane has 28 carbon atoms.
Octadecane contains 18 carbon atoms. It is used as a solvent, lubricant transformer oil, and anti-corrosion agent.
Octaheptacontane contains 78 carbon atoms.
Octahexacontane is the longest carbon chain that has 68 carbon atoms.
Octane contains eight carbon atoms. Octane is a colorless and flammable hydrocarbon present in the petroleum spirit.
Octanonacontane has 98 carbon atoms.
Octaoctacontane has 88 carbon atoms.
Octapentacontane contains 58 carbon atoms.
Octatetracontane contains 48 carbon atoms.
Octatriacontane contains 38 carbon atoms.
Pentacontane contains 50 carbon atoms. It is a saturated hydrocarbon compound.
Pentacosane consists of 25 carbon atoms. Pentacosane has a great role as a semiochemical and plant metabolite.
Pentadecane is an alkane hydrocarbon consisting of 15 carbon atoms. Pentadecane is used in organic synthesis and as a solvent.
Pentaheptacontane has 75 carbon atoms. Pentaheptacontane is a saturated hydrocarbon that has no carbon-carbon multiple bonds.
Pentahexacontane contains 65 carbon atoms. It is a colorless and flammable liquid alkane derived from petroleum.
Pentane is a hydrocarbon with five carbon atoms. Pentane has various industrial uses including it is used to create a blowing agent which is used to create a foam known as polystyrene.
Pentanonacontane has 95 carbon atoms.
Pentaoctacontane has 85 carbon atoms.
Pentapentacontane has 55 carbon atoms.
Pentatetracontane has 45 carbon atoms.
Pentatriacontane has 35 carbon atoms. It is an acyclic branched or unbranched hydrocarbon, and contains entirely hydrogen atoms and saturated carbon atoms.
Tetracontane consists of 40 carbon atoms. Tetracontane is used as a prototype of linear polyethylene to understand the phase behavior of polymer solution.
Tetracosane has 24 carbon atoms. It has great roles as a plant metabolite and a volatile oil component.
Tetradecane consists of 14 carbon atoms. It is used in lubricants and greases, adhesives and sealants, fingerprints, and more.
Tetraheptacontane has 74 carbon atoms.
Tetrahexacontane consists of 64 carbon atoms.
Tetranonacontane has 94 carbon atoms.
Tetraoctacontane contains 84 carbon atoms.
Tetrapentacontane consists of 54 carbon atoms.
Tetratetracontane consists of 44 carbon atoms.
Tetratriacontane has 34 number of carbons in it.
Triacontane has 30 carbon atoms. Triacontanes are present in petroleum products.
Tricosane contains 23 carbon atoms.
Tridecane has 13 carbon atoms.
Triheptacontane consists of 73 carbon atoms.
Trihexacontane has 63 carbon atoms.
Trinonacontane contains 93 carbon atoms.
Trioctacontane has 83 carbon atoms.
Tripentacontane has 53 carbon atoms.
Tritetracontane has 43 carbon atoms.
Tritriacontane is an unbranched long chain alkane. The number of carbon atoms is 33.
Undecane is a liquid alkane hydrocarbon that has 11 carbon atoms.
Carbon Chain Group Names
Carbon chains belong to groups depending on their physical and chemical properties. Go through the following carbon chain length names and understand their group names.
Alcohols are a type of organic compound that carries at least one hydroxyl functional group (-OH). Carbon chain names alcohols are referred to as the primary alcohol ethanol which is used as a drug, and it is the main alcohol present in the alcohol drinks.
Aldehydes are organic compounds consisting of functional groups with the structure -C(H)=O. Aldehydes play an important role in the technology and biological spheres.
Alkanes are acyclic saturated hydrocarbons and they contain entirely hydrogen atoms and saturated carbon atoms.
Alkenes and Alkynes - Alkenes are unsaturated hydrocarbons containing at least one carbon-carbon double bond. Alkynes are also unsaturated hydrocarbons containing at least one carbon-carbon triple bond between two carbon atoms. Alkenes are more reactive than their related chain alkanes, this is because of the relative instability of the pi bond.
Alkyl halides are compounds in which one or more hydrogen atoms in an alkane are replaced by halogen atoms like fluorine, chlorine, bromine, and iodine. They fall into different classes on the basis of how the halogen atom is positioned on the chain of carbon atoms.
Alkyl halides can be categorized as primary, secondary, and tertiary. Alkyl groups are one among the carbon chain group names.
Amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. They are derivatives of ammonia and one or more hydrogen atoms in it have been replaced by a substituent, such as an alkyl or aryl group.
Carboxylic acids are organic acids that contain a carboxyl group (C(=O)OH) attached to an R- group. R refers to the alkyl, alkenyl, aryl, or other groups. Carboxylic acids occur widely.
Esters are chemical compounds derived from organic or inorganic acid. In esters, at least one -OH hydroxyl group is replaced by an -O- alkyl (alkoxy) group.
Ethers are a class of organic compounds that contain an ether group which is an oxygen atom linked to two alkyl and aryl groups. The general formula of ethers is R-O- R.
Ketones: In organic chemistry, a ketone is a functional group that contains a variety of carbon-containing substituents. It contains a carbonyl group which is a carbon-oxygen double bond. Ketones play an important role in biology and in industry.
Important Carbon Chain Names
There exist various carbon chains in nature. Some of them are very important as they have importance in the day-to-day life of human beings. Look through the following list of important carbon chain names and explore their features.
Butane, which has four carbon atoms, is a colorless gas and has a faint petroleum-like odor.
Decane consists of 10 carbon atoms.
Ethane is a gaseous and colorless hydrocarbon containing two carbon atoms and six hydrogen atoms. It is the simplest hydrocarbon structurally as it contains a single carbon-carbon bond.
Heptacontane is a 70 carbon-containing hydrocarbon.
Hexacontane consists of 60 carbon atoms.
Hexadecane contains 16 carbon atoms.
Hexaheptacontane contains 76 carbon atoms.
Hexanonacontane contains 96 carbon atoms.
Hexatetracontane contains 46 carbon atoms.
Hexatriacontane contains 36 carbon atoms.
Icosane has 20 carbon atoms.
Methane is the simplest hydrocarbon containing one carbon atom and four hydrogen atoms. Methane is important as it is a very potent and important greenhouse gas.
Nonacontane contains 90 carbon atoms.
Nonane has nine carbon atoms.
Nonanonacontane contains 99 carbon atoms.
Octacontane consists of 80 carbon atoms.
Octane contains eight carbon atoms.
Octanonacontane has 98 carbon atoms.
Octaoctacontane has 88 carbon atoms.
Octatetracontane contains 48 carbon atoms.
Octatriacontane has 38 carbon atoms.
Pentacontane has 50 carbon atoms.
Pentacosane consists of 25 carbon atoms.
Pentadecane is an alkane hydrocarbon consisting of 15 carbon atoms.
Pentaheptacontane has 75 carbon atoms.
Pentahexacontane contains 65 carbon atoms.
Pentane is a hydrocarbon with five carbon atoms.
Pentanonacontane has 95 carbon atoms.
Pentaoctacontane has 85 carbon atoms.
Pentapentacontane has 55 carbon atoms.
Pentatetracontane has 45 carbon atoms.
Pentatriacontane has 35 carbon atoms.
Propane is another gaseous hydrocarbon containing three carbon atoms and eight hydrogen atoms.
Tetracontane consists of 40 carbon atoms.
Tetracosane has 24 carbon atoms.
Tetrapentacontane consists of 54 carbon atoms.
Triacontane has 30 carbon atoms.
We Want Your Photos!
More for You
Master of Computer Science

Abhijeet ModiMaster of Computer Science
An experienced and innovative entrepreneur and creative writer, Abhijeet holds a Bachelor's and Master's degree in Computer Application from Birla Institute of Technology, Jaipur. He co-founded an e-commerce website while developing his skills in content writing, making him an expert in creating blog posts, website content, product descriptions, landing pages, and editing articles. Passionate about pushing his limits, Abhijeet brings both technical expertise and creative flair to his work.
Bachelor of Arts specializing in English Literature

Naman KhannaBachelor of Arts specializing in English Literature
An English literature graduate from Delhi University, Naman's broad interests include mathematics, science, and social science. With his knowledge and expertise in multiple subjects, he is an asset to our fact-checking team. Naman is set to pursue his postgraduate degree in English literature soon.
Disclaimer
1) Kidadl is independent and to make our service free to you the reader we are supported by advertising. We hope you love our recommendations for products and services! What we suggest is selected independently by the Kidadl team. If you purchase using the Buy Now button we may earn a small commission. This does not influence our choices. Prices are correct and items are available at the time the article was published but we cannot guarantee that on the time of reading. Please note that Kidadl is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon. We also link to other websites, but are not responsible for their content.
2) At Kidadl, we strive to recommend the very best activities and events. We will always aim to give you accurate information at the date of publication - however, information does change, so it’s important you do your own research, double-check and make the decision that is right for your family. We recognise that not all activities and ideas are appropriate for all children and families or in all circumstances. Our recommended activities are based on age but these are a guide. We recommend that these ideas are used as inspiration, that ideas are undertaken with appropriate adult supervision, and that each adult uses their own discretion and knowledge of their children to consider the safety and suitability. Kidadl cannot accept liability for the execution of these ideas, and parental supervision is advised at all times, as safety is paramount. Anyone using the information provided by Kidadl does so at their own risk and we can not accept liability if things go wrong.
3) Because we are an educational resource, we have quotes and facts about a range of historical and modern figures. We do not endorse the actions of or rhetoric of all the people included in these collections, but we think they are important for growing minds to learn about under the guidance of parents or guardians.